Signal transduction in the endothelium-group
Group leader: Anita Boratkó, Ph.D. (boratko@med.unideb.hu)
Group members:
Assala Raya, Ph.D student
Fanni Szalmás, Ph.D student
Márton Fonódi, Ph.D student
Inez Pivnyik, M.Sc student
Perez Santamaria Fernanda Estefania, M.Sc student
Tanisha Mohanthy, MD student
Karolina Kovács, laboratory assistant
Research background:
The endothelium serves as a crucial barrier between blood and surrounding tissues, maintaining vascular integrity and regulating the exchange of substances such as nutrients and oxygen. It plays a pivotal role in angiogenesis, the process of new blood vessel formation, which is essential for tissue growth and repair. Endothelial cells actively participate in immune responses by controlling leukocyte adhesion and trafficking across blood vessel walls. They also contribute to hemostasis by regulating blood clotting and fibrinolysis processes. Furthermore, endothelial cells secrete various factors involved in vascular tone regulation, influencing blood pressure and blood flow distribution throughout the body. Damage to the endothelial barrier or its dysfunction can be observed during inflammatory processes, trauma, sepsis, diabetes mellitus, thrombosis, or the formation of metastatic tumors. Endothelial cells are recruited to form new blood vessels that supply nutrients and oxygen to tumors. This supports tumor growth and metastasis, making it a target for anti-cancer therapies aimed at inhibiting new blood vessel formation. Due to the extremely large surface area of the pulmonary vasculature, the balance maintained by the pulmonary endothelium between the lumen and the interstitium is very sensitive to dynamic changes in endothelial permeability. Thus, for example, during the development of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), due to damage to the endothelium, the alveoli are unable to exchange oxygen and carbon dioxide, and edema develops due to the disturbance of fluid exchange between capillaries and tissues. In addition to the loss/reduction of organ function, the leakage of blood vessels is also significant from a pharmacokinetic point of view.
We study Ser/Thr specific protein phosphatases in connection with the barrier function of pulmonary artery endothelial cells. We investigate the regulation, function, possible substrates, regulatory subunits and protein interaction partners of protein phosphatase 1 and 2A enzymes in pulmonary artery endothelial cells in order to better understand the physiological function of vascular endothelial cells. Regulatory subunits play a primary role in the regulation of enzymes, so in our current research we are examining two specific phosphatase regulatory subunits. Our work so far confirms that the coordinated reversible phosphorylation of several proteins through different signal transduction processes plays a regulatory role in several endothelial function.
Current research projects
- Investigation of the TIMAP protein, a protein phosphatase 1 regulatory subunit in endothelial cells
TIMAP (TGF-β inhibited membrane-associated protein) has a high expression level in endothelial cells, but little is known about its function and interacting partners. Based on its structural characteristics, it is considered a regulatory subunit of protein phosphatase 1, a member of the myosin phosphatase regulator (MYPT) family. The main research profile of our working group is the investigation of the role of the TIMAP protein in endothelial cells. We have shown that TIMAP regulates the endothelial barrier by dephosphorylation of ezrin-radixin-moesin proteins and participates in angiogenesis through the endothelin signaling pathway. Several new TIMAP-PP1 complex substrate proteins were identified (e.g. neurofibromin 2, eukaryotic elongation factor A2A). With our research, we came closer to discovering the post-translational regulation of TIMAP (new phosphorylation sites and prenylation mechanism). The research results may open new horizons in the identification of potential pharmacological targets for diseases associated with endothelial dysfunction, through signaling mechanisms that have not been explored so far.
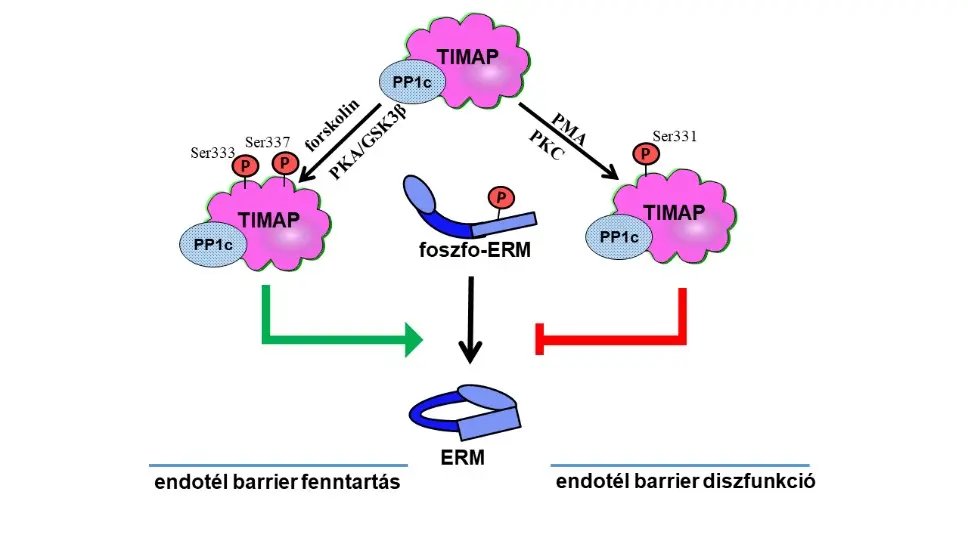 2. Investigation of the TIMAP protein in neuronal cells
2. Investigation of the TIMAP protein in neuronal cells
TIMAP is also expressed in neuronal cells, but details of its function have not been studied yet. We aimed to explore the role of TIMAP in neuronal cells, especially during differentiation. We showed that TIMAP–PP1c complex is present in neuronal cells, and TIMAP appears to be an inhibitor of neuronal differentiation. We found that TIMAP translocated to the nuclear region of cells in differentiated cells. The nuclear interactome of TIMAP revealed several new potential protein binding partners, but the detailed signaling pathways remain to be investigated. These binding partners can be novel substrates of TIMAP–PP1c and further demonstrate the possible role of TIMAP as a transcriptional regulator.
- The role of the protein phosphatase 2A B55 regulatory subunit containing holoenzyme in the physiological processes of endothelial cells
PP2A holoenzyme is a heterotrimeric complex composed of three main subunits (subunit A, B, C). The B regulatory subunit confers substrate specificity and regulates the activity of PP2A by binding to specific protein targets. PP2A holoenzyme functions as a major cellular phosphatase that counteracts the actions of protein kinases. It regulates diverse cellular processes such as cell cycle progression, apoptosis, metabolism, and signal transduction pathways. During our research, we examined the role of the PP2A-B55 holoenzyme in the dephosphorylation of proteins responsible for cell-cell interactions, which regulates the barrier function of endothelial cells. We identified the EBP50 protein, the dephosphorylation of which is important during mitosis, as a substrate protein, as well as the flotillin-1 protein, further confirming the role of PP2A in angiogenesis. Recently, we showed evidence for the intracellular role of PP2A B55α in angiogenesis, specifically through the regulation of thrombospondin-1 (TSP1), a well known angiogenesis inhibitor. we introduced a new regulatory mechanism of angiogenesis by reversible phosphorylation on TSP1 by protein kinase C and PP2A B55α holoenzyme.
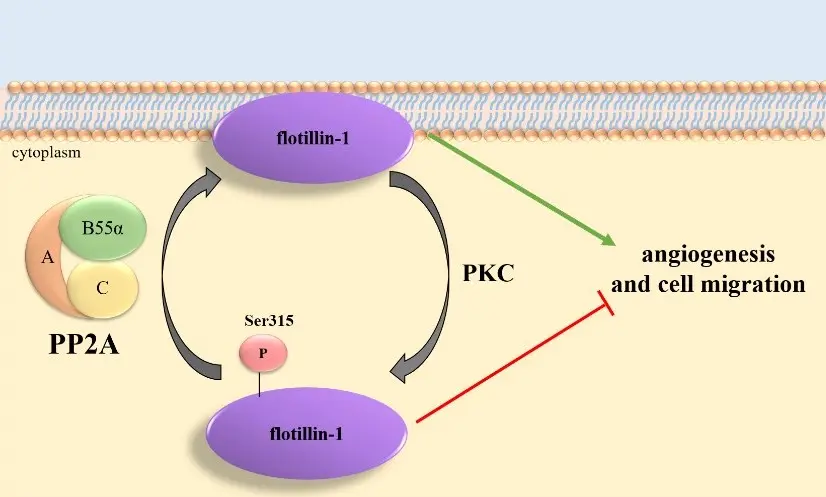
Recent publications
PP2A Affects Angiogenesis via Its Interaction with a Novel Phosphorylation Site of TSP1.
Thalwieser Z, Fonódi M, Király N, Csortos C, Boratkó A.
Int J Mol Sci. 2024 Feb 3;25(3):1844. doi: 10.3390/ijms25031844.
PMID: 38339122
TIMAP, a Regulatory Subunit of Protein Phosphatase 1, Inhibits In Vitro Neuronal Differentiation.
Fonódi M, Thalwieser Z, Csortos C, Boratkó A.
Int J Mol Sci. 2023 Dec 11;24(24):17360. doi: 10.3390/ijms242417360.
PMID: 38139189
Dephosphorylation of annexin A2 by protein phosphatase 1 regulates endothelial cell barrier.
Király N, Thalwieser Z, Fonódi M, Csortos C, Boratkó A.
IUBMB Life. 2021 Oct;73(10):1257-1268. doi: 10.1002/iub.2538. Epub 2021 Aug 9.
PMID: 34331392
Protein phosphatase 2A-mediated flotillin-1 dephosphorylation up-regulates endothelial cell migration and angiogenesis regulation.
Thalwieser Z, Király N, Fonódi M, Csortos C, Boratkó A.
J Biol Chem. 2019 Dec 27;294(52):20196-20206. doi: 10.1074/jbc.RA119.007980. Epub 2019 Nov 21.
PMID: 31753918