Group leader:
Magdolna Szántó PharmD, PhD
Members:
Bailasan Ahmad Hassan, PhD student
Virág Kovács, BSc student
Background
Immunometabolism is an emerging concept in explaining the underlying mechanisms of several inflammatory diseases, among them psoriasis and eczema, two of the most common inflammatory skin disorders. Although psoriasis and eczema are mainly immune-mediated diseases, the direct correlation between obesity and severity of both psoriasis and eczema implies that metabolic derangements and a consequent reprogramming of immune cells may be a major event in the manifestation of the diseases. Thus, such interventions that are able to interrupt relevant metabolic signals may have the potential to simultaneously reduce inflammatory burden in the skin, and decrease the risk of exacerbation of the diseases and the development of systemic metabolic complications, without the undesired effects of immunosuppressive biologicals.
In our work, our approach to the management of psoriasis and eczema is unique in several respects. We propose immunoresolving over immunosuppression, by targeting the pathogenic metabolic programs of immune cells without affecting normal immune cell functions. We propose this by nominating members of a well-known enzyme family, poly(ADP-ribose) polymerases (PARPs), as potential targets in inflammatory skin diseases.
PARPs catalyse the ADP-ribosylation reaction that is a phylogenetically conserved posttranslational modification. ADP-ribosylation was originally linked to DNA damage repair, but now it is widely acknowledged that it is involved in a plethora of fundamental processes, including regulation of chromatin structure, transcriptional control, cell death, cell proliferation and differentiation, host defense, energy homeostasis, metabolic regulation and lipid metabolism.
Two decades ago it was recognised that tumour cells with defective homologous recombination DNA repair pathways are highly sensitive to the inhibition of PARP activity, which triggered extensive drug development efforts. As a result, currently five PARP inhibitors have FDA approval for clinical use in tumour therapy. In parallel, another concept emerged with regard to the therapeutic potential of PARP inhibitors. As PARPs use NAD+ as substrate, the overactivation of PARPs may deplete cellular NAD+ content, and hence is commonly associated with metabolic disturbances, and a pro-inflammatory state. Obesity is associated with increased PARP activity in white adipose tissue, and PARP inhibition reduced obesity and improved metabolic functions in mice. In accordance, the anti-inflammatory effect of PARP inhibition has long been recognized. Interestingly, psoriatic patients display elevated lesional PARP activity and reduced serum NAD+ level.
We have previously shown that PARPs regulate the inflammatory pathways involved in the patomechanism of psoriasis. Based on our previous studies we hypothesize that the activation of PARPs contributes to the reprogramming of immune cells under abnormal metabolic conditions, which may be relevant in psoriasis (and in eczema) pathomechanism and the development of psoriasis-associated metabolic comorbidities. The primary objective of our work is to determine the roles of PARPs in the immunometabolism of psoriasis as well as of eczema, and to evaluate PARP inhibitor efficacy in inflammatory skin diseases in preclinical settings.
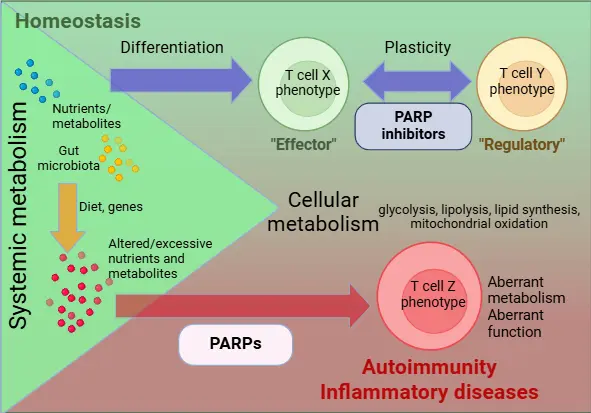
It is important that our results may offer far-reaching benefits beyond psoriasis. There are several inflammatory diseases (such as atopic dermatitis and asthma), which are characterized by PARP overactivation, as well as display a close correlation with metabolic disorders. Our aim is to provide a comprehensive analysis on how PARPs may regulate the adaptation of immune cells to metabolic alterations. These data may highlight a new role of PARPs in inflammation, and may further the conduction of clinical studies with PARP inhibitors in novel non-oncological indications. Given that PARP inhibitors are already used in clinical therapy, our results may have immediate translational potential.